This articel introduces a highly efficient method for refining quartz powder, which is suitable for the manufacture of quartz glass containers and drill moulds necessary for semiconductor industry. The high purity raw material is also suitable for adding silica filler into plastic packaging.
Production problem of refining quartz powder
Quartz powder, especially natural quartz powder, has been used as raw material of quartz glass to manufacture quartz glass containers and drill tools, and as silica filler added to plastic packaging, such as IC and LSIS. However, it has been found that these quartz powders contain various impurities, and these impurities have adverse effects on semiconductor products.
Produce high-purity quartz powder at low cost
Sinonine carried out in-depth research on related technologies and found that
high-purity quartz powder, especially high-purity quartz powder without alkali metal elements, alkali earth metal elements and transition metal elements, can be prepared at low cost by installing at least three chambers in the rotary quartz tube, namely a preheating chamber, a reaction chamber and a gas desorption chamber. The quartz powder material is transferred from one cavity to another continuously. The production process can achieve two purposes: providing a continuous refining method of quartz powder, which can completely remove the impurities that will affect the silicon wafer from the quartz powder; and providing a refining method of quartz powder which can produce high-purity quartz powder at low cost.

The quartz powder described here contains a powder consisting of particles with diameters ranging from 106 to 250 microns. It is obtained by conventional particle size reduction process and grading of natural quartz, crystal, siliceous rock or artificial quartz. In fact, quartz can be heated to promote particle size reduction. Fine quartz powder consisting of particles less than the limited range is not suitable for making quartz glass; on the contrary, quartz powder consisting of particles larger than the above range can not be fully purified rather than optimized.
The segmented plate is made of quartz glass. The relative aperture of the holes on the plate depends on the size and quality of the starting material of quartz powder, so that the ratio is in the range of 10% to 40% of the total area of the segmented plate. In particular, the pore-to-diameter ratio is optimally selected within the range of basically blocked quartz powder whose pore is reversed in the refining process.
The above-mentioned "overturned quartz powder is basically blocked" means that the hole is closed by quartz powder, by which chlorine gas can not flow into the gas desorption chamber from the effective cross section of the hole. By maintaining this state, chlorine-containing gas molecules can be prohibited from re-adhering to purified quartz powder. In this way, higher refining efficiency can be obtained.
The inlet and outlet of the starting material and the exhaust outlet of the chlorine gas are arranged on the preheating chamber of the converter. The inlet of the pipeline for supplying the reaction gas is arranged in the reaction chamber to input the chlorine-containing gas. The exhaust holes of desorption gas and quartz powder products are arranged in the desorption chamber. In this way, the refined quartz powder can be removed when the desorption gas is discharged. Vacuum extraction equipment can be connected with the desorption gas discharge pipeline to accelerate gas desorption.
The preheating chamber is heated to 800 ℃ and transferred to the reaction chamber by feeding the quartz powder into the converter through the material feeding tube. The quartz powder was then purified by contacting chlorine-containing gas from the reaction gas intake pipe in the temperature range of 1000 to 1300 ℃. The purified quartz powder is turned over and transferred into a gas desorption chamber heated to at least 800 C to release the absorbed gas from the surface of the quartz powder, and the hot quartz powder is gradually cooled.
In the above refining process, if the temperature of the preheating chamber is lower than 800 ℃, the purification reaction in the reaction chamber can not proceed smoothly. If the temperature of the reaction chamber is lower than 1000 ℃, the purification efficiency will be reduced. In this case, the desired purity will not be achieved. If the temperature of the reaction chamber exceeds 1300 C, all the equipment will not be able to withstand. Before entering the reaction chamber, chlorine-containing gases should be pre-heated to near the reaction temperature. In this way, the cooling of quartz powder can be avoided and high purification efficiency can be maintained. The chlorine-containing gas can pass through, for example, the reaction chamber directly after being heated outside the converter. On the other hand, before entering the reaction chamber, it can heat chlorine-containing gas in the gas desorption chamber through the reaction gas intake pipe arranged in the gas desorption chamber.
The chlorine-containing gases of the quartz sand refining method include a mixture of gaseous hydrogen chloride and gaseous chlorine, or a mixture of gases with inert gases such as nitrogen. In particular, it is preferable to mix gaseous hydrogen chloride with gaseous chlorine at a mixing ratio of 2 to 20, preferably between 4 and 13. If the mixing ratio of gaseous hydrogen chloride and gaseous chlorine is reduced beyond the above range, alkaline earth metal elements and other transition metal elements will not be fully removed from quartz powder.
The refining speed is in the range of 1 kg/h to 20 kg/h, and the optimum is 1 to 10 kg/h. If the velocity is too low to be below this range, alkali metal elements cannot be removed from quartz powder very quickly. In order to improve the purification efficiency, the refining steps of the present invention can be repeated many times. This method is especially suitable for improving the efficiency of removing transition metal elements from quartz powder.
The refining method of quartz sand is suitable for preparing pure quartz powder without alkali metal elements, transition metal elements and alkali earth metal elements. Conventional processes and methods can not adequately remove alkaline earth metal elements. More specifically, the quartz powder provided by the method of the present invention contains sodium with a concentration as low as 10 ppb or lower, iron with a concentration of 70 ppb or lower, copper and nickel with a concentration of 0.3 ppb or lower, and chromium with a concentration of 0.5 ppb or lower, respectively.
The good result of quartz powder device
The preheating chamber 1, the reaction chamber 2 and the gas desorption chamber 3 are arranged in the quartz glass converter 4 with the inner diameter of 100mm and the length of 2000m. The three chambers 1, 2 and 3 are separated by quartz glass segmented plates 5 and 6. The pore 7 is set on the segment plate 5 between the preheating chamber 1 and the reaction chamber 2, and the pore diameter ratio is 15%. The other hole 8 is set on the segment plate 6 between the reaction chamber 2 and the gas desorption chamber 3, and the pore diameter ratio is 35%. The converter is placed in heater 9 at an inclined angle of 4 degrees. Heater 9 is divided into three heating zones as shown in vertical line 19.
Natural quartz powder 10 with particle diameter of 106-250 um is continuously fed to converter 4 through feeding silo 11, and its speed exceeds about 1.5 kg per hour. In the process of feeding quartz powder 10, converter 4 rotates around its axis 12. In the preheating chamber 1, quartz powder 10 is heated to about 1000 C by a heater 9 placed outside converter 4. The gas desorber 18 is inserted into the preheating chamber 1 to remove toxic gases that may exist in the preheating chamber 1. The exhaust flow direction is indicated by arrow 20.
The heated quartz powder 10 is reversed and transferred into the reaction chamber 2 through the segmented plate 5. The segment plate 5 is made into a ring with holes in the center and the outer diameter less than the inner diameter of converter 4. Thus, there is a gap between the inner walls of the segment plate 5 and converter 4. Quartz powder 10 can be transferred to the reaction chamber 2 through the middle hole and the gap.
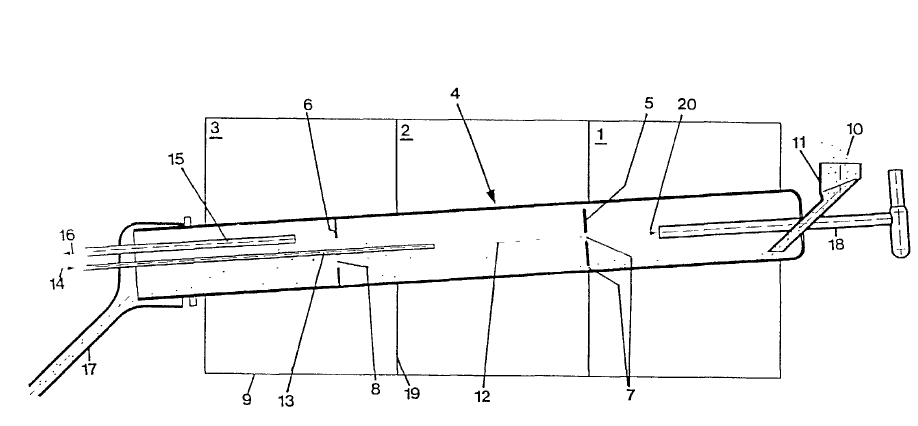
In reaction chamber 2, quartz powder 10 is further heated to about 1270 C. At the same time, quartz powder 10 was purified by contacting with the mixture, which contained gaseous hydrogen chloride and chlorine. The flow rate ratio of the former to the latter was 1-0.0751/min (13:1). The mixture gas passes through the tube 13 to the reaction chamber 2, and the tube 13 extends from one end of converter 4 to the reaction chamber 2 along the central axis through the middle hole 8 on the section plate 6. The flow direction of the mixture is indicated by arrow 14.
In this way, the purified quartz powder 10 is transferred to the gas desorption chamber 3 whose temperature is kept at 800 C, and gradually cooled to remove the gas adsorbed on the surface of quartz powder 10. The exhaust pipe 15 of the desorption gas is arranged on the gas desorption chamber 3, and the ventilation equipment can be connected with the exhaust pipe 15 to accelerate the desorption. The flow direction of the exhaust gas is indicated by arrow 16.
Quartz powder 10 is refined continuously through the gas desorption chamber through the discharge hole connected with the discharge tube 17, and 10 kg high purity quartz powder 10 is obtained for 2 hours. The purified quartz powder was analyzed by chemical method. The results are as follows.

Refined quartz powder technology is a high-tech, sinonine is in the leading level in the industry.





